A real-time, point-of-care, wearable heparin sensor: Pediatric catheter dosimetry using photoacoustic imaging
Background
Heparin anticoagulation therapy is a cornerstone of surgical and cardiovascular medicine because of its short half-life, reversible nature, and low cost, but it also suffers from a narrow therapeutic window and is the second most common medication error. It is used prophylactically in pediatric patients undergoing angiography, bypass, cannulation, extracorporeal membrane oxygenation, as well as therapeutically in thromboses, and cancer. Heparin is used in 15% of pediatric inpatients, and approximately one in seven patients has an adverse event, for an annual morbidity of 140,000; mortality rates of the diseases treated with heparin are 2-20% and include cerebral sinovenous thrombosis and vascular thromboembolism. These inherent challenges are compounded by iatrogenic errors such as incorrect heparin ordering, inaccurate infusion pump settings, and incomplete record keeping, resulting in ~10,000 heparin medication errors per year. Heparin is involved in more medication errors than morphine and vancomycin with multiple deaths annually particularly in neonates. Thousands more suffer from hemorrhages or emboli because of inaccurate dosing and dosing/monitoring programs that are designed for adult populations. Because of these issues, heparin anticoagulation therapy must be monitored very carefully. The current standard of care is the activated partial thromboplastin time; however, this in vitro diagnostic tool requires large blood volumes and suffers from long turnaround times, a variable pediatric reference range, and poor correlation to heparin dose/patient performance. Clearly, there is an unmet need for a real-time and non-invasive tool to monitor heparin.1The field of pediatric coagulation are desperate for new and improved analytical instrumentation to increase the efficacy of heparin monitoring.
Technology Description
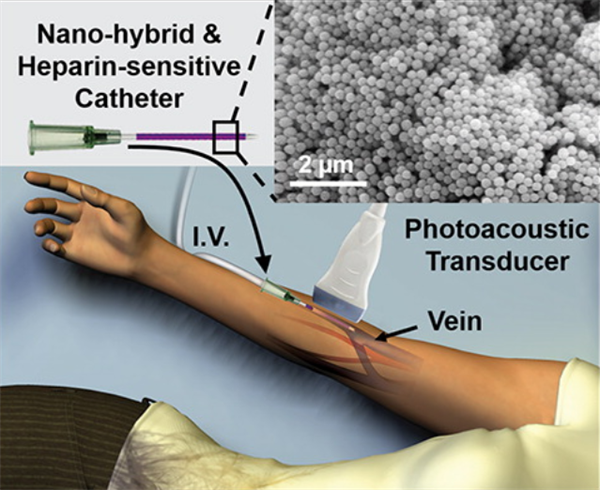
Researchers from UC San Diego have developed a peripheral venous catheter/cannula that will measure in vivo heparin levels via a non-invasive ultrasound photoacoustic imaging technique. More specifically, using this technique with methylene blue produces a dose-dependent signal in the presence of heparin and is ideal for monitoring drugs, like heparin, with a narrow therapeutic window.
Related Materials
Patent Status
| Country | Type | Number | Dated | Case |
| United States Of America | Published Application | 20190029637 | 01/31/2019 | 2016-166 |
| Patent Cooperation Treaty | Published Application | 2017132642 | 08/03/2017 | 2016-166 |
Contact
- University of California, San Diego Office of Innovation and Commercialization
- innovation@ucsd.edu
- tel: View Phone Number.
Other Information
Keywords
acoustic sensor, anticoagulation therapy, cannula, catheter, catheter cladding, heparin, partial thromboplastin time, PTT, smart catheter, wearable sensor
